Effect of Ammonia on the Formation of THMS in Drinking Water Chlorination - A Case Study
DOI:
https://doi.org/10.5281/zenodo.17015630Keywords:
ammonia, chlorination, drinking water, natural organic matter (nom), thms formationAbstract
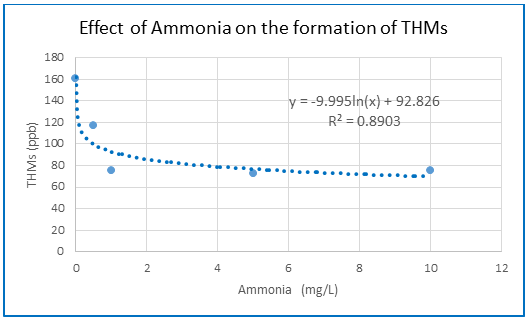
In a water supply system total Trihalomethanes (THMs) content in drinking water may vary considerably depending on water quality and treatment conditions. Most urban water treatment plants generally use chlorine as disinfectant. The effect of various parameters on the formation of THMs has been widely studied around the world over the past few decades. Almost universally, it has been found that increasing any of these parameters tends to promote the formation of THMs—except for ammonia, which has a negative effect on the process. Surprisingly, this exception has not received the attention it deserves in THM research globally. Given the high concentration of ammonia in Dhaka's drinking water sources—particularly during the dry months—this study aimed to evaluate how ammonia affects the formation of THMs in water samples from the largest water treatment plant in Dhaka, Bangladesh. The water samples were tested for a wide range of parameters including pH, ammonia, UV254, TOC, DOC and bromide following the standard methods of testing. THMs was measured by THM plus Method (Method:10132) using UV-VIS Spectrophotometer DR 6000(HACH, USA). A detailed quantitative study was conducted to examine how ammonia affects the formation of trihalomethanes (THMs) when water is chlorinated under varying conditions. Experiments were carried out using treated water from the supply system, which had a dissolved organic carbon (DOC) content of 6.0 mg/L. Chlorination was performed with a free chlorine residual of 0.89 mg/L and a total chlorine residual of 1.29 mg/L. Different doses of ammonia—0.0, 0.5, 1.0, 5.0, and 10.0 mg/L—were applied. The results showed that the presence of ammonia at various concentrations significantly reduced THM formation at the given chlorine levels, however it did not completely eliminate it. THMs formation decreased continuously with increasing ammonia concentration, and the decline is sharp during relatively low concentration of ammonia up to 3 mg N/L then remained near to flat slope after ammonia exceeded 3 mg N/ L. It is noticed that the formation of THMs significantly reduced with increasing ammonia concentration from 0 to 10 mg N/L in chlorinated drinking water. The suppression of THMs was prominent with increasing ammonia concentration from almost zero to 5 mg N/L. However, the formation of THMs remain low and constant after ammonia addition over 5 mg N /L. A general correlation for predicting THM formation based on ammonia concentration is presented, and its predictions align well with the observed results, although it is specific to this study. Further research using a more diverse dataset is recommended. In water supply systems like Dhaka, where significant amounts of ammonia and other organic pollutants are present in river water—along with the perceived risk of THM formation—comprehensive studies should be undertaken to determine how to manage or utilize ammonia effectively during water treatment. One potential approach could be the controlled use of ammonia to form chloramine, which can act as a disinfectant in the treatment process.
Downloads
References
ACC. (2018). Drinking water chlorination: A review of U.S. disinfection practices and issues. Chlorine Chemistry Division, American Chemistry Council.
Adams, C., Timmons, T., Seitz, T., Lane, J., & Levotch, S. (2005). Trihalomethane and haloacetic acid disinfection by-products in full-scale drinking water systems. Journal of Environmental Engineering, 131(4), 526-534.
Amy, G. L., Chadik, P. A., & Chowdhury, Z. K. (1987). Developing models for predicting trihalomethane formation potential and kinetics. Journal‐American Water Works Association, 79(7), 89-97.
Amy, G. L., Chadik, P. A., King, P. H., & Cooper, W. J. (1984). Chlorine utilization during trihalomethane formation in the presence of ammonia and bromide. Environmental science & technology, 18(10), 781-786.
Bellar, T.A., Lichtenberg, J.J., & Kroner, R.C. The occurrence of organohalides in chlorinated drinking waters. Jour. AWWA, 66(12), 703.
Bittner, A. A. B. (2000). Nepal drinking water quality assessment: Nitrates and ammonia. (Doctoral Dissertation, Massachusetts Institute of Technology).
Black, B. D., Harrington, G. W., & Singer, P. C. (1996). Reducing cancer risks by improving organic carbon removal. Journal‐American Water Works Association, 88(6), 40-52.
Bujar, H. D. (2013). The study of the trihalomethanes (THMs) content variation with advanced analytical methods in the drinking water in the city of Tetova-Studimi i variacionit të përmbajtjes së trihalometaneve (THM) në ujin e pijshëm të ujësjellësit të qytetit të Tetovës me metoda analitike të përparuara. (Doctoral Dissertation, Dissertation, University of Tirana, Albania, pp. 3-15, 23-28).
Carlson, M., & Hardy, D. (1998). Controlling DBPs with monocholoramine. Journal‐American Water Works Association, 90(2), 95-106.
Chen, C., Zhang, X. J., Zhu, L. X., Liu, J., & He, W. J. (2008). Disinfection by-products and their precursors in a water treatment plant in North China: seasonal changes and fraction analysis. Science of the Total Environment, 397(1-3), 140-147.
Chowdhury, S., Champagne, P., & Husain, T. (2007). Fuzzy risk-based decision-making approach for selection of drinking water disinfectants. Journal of Water Supply: Research and Technology - AQUA, 56(2), 75-93.
Clark, R. M., Adams, J. Q., Sethi, V., & Sivaganesan, M. (1998). Control of microbial contaminants and disinfection by-products for drinking water in the US: Cost and performance. Journal of Water Supply: Research and Technology - AQUA, 47(6), 255-265.
Clark, R. M., Thurnau, R. C., Sivaganesan, M., & Ringhand, P. (2001). Predicting the formation of chlorinated and brominated by-products. Journal of Environmental Engineering, 127(6), 493-501.
Cowman, G. A., & Singer, P. C. (1995). Effect of bromide ion on haloacetic acid speciation resulting from chlorination and chloramination of aquatic humic substances. Environmental Science & Technology, 30(1), 16-24.
DoE. (2023). The environment conservation rules 1997. Government of the People’s Republic of Bangladesh, Dhaka.
Duong, H. A., Berg, M., Hoang, M. H., Pham, H. V., Gallard, H., Giger, W., & von Gunten, U. (2003). Trihalomethane formation by chlorination of ammonium-and bromide-containing groundwater in water supplies of Hanoi, Vietnam. Water Research, 37(13), 3242-3252.
EECD. (1997). Amended proposal for a Council Directive concerning the quality of water intended for human consumption-common position. in Proc. Council of the European Union, Directive 80/778/EEC, Com (97) 228, Final 95/0010 SYN, Brussels.
EPA. (1979). National interim primary drinking water regulations: Control of trihalomethanes in drinking water. Final Rule.
Engerholm, B. A., & Amy, G. L. (1983). A predictive model for chloroform formation from humic acid. Journal‐American Water Works Association, 75(8), 418-423.
Fayyad, M. K., & Al-Sheikh, A. M. (2001). Determination of N-chloramines in As-Samra chlorinated wastewater and their effect on the disinfection process. Water research, 35(5), 1304-1310.
Federal-Provincial Subcommittee on Drinking Water (Canada). (1996). Guidelines for Canadian drinking water quality. Canadian Government Publishing.
Fu, Q., Zheng, B., Zhao, X., Wang, L., & Liu, C. (2012). Ammonia pollution characteristics of centralized drinking water sources in China. Journal of Environmental Sciences, 24(10), 1739-1743.
Gang, D. D., Segar Jr, R. L., Clevenger, T. E., & Banerji, S. K. (2002). Using chlorine demand to predict TTHM and HAA9 formation. Journal‐American Water Works Association, 94(10), 76-86.
Karen, L.S., Keith, P.H. (1998). Drinking water disinfection by-products: an Australian perspective. Water Res., 32, 1522–1528.
King, W. D., Marrett, L. D., & Woolcott, C. G. (2000). Case-control study of colon and rectal cancers and chlorination by-products in treated water. Cancer Epidemiology and Prevention Biomarkers, 9(8), 813-818.
Motasem, S., Khaled, R., &Manar, F. (2013). Investigation of factors affecting THMs formation in drinking water. American Journal of Environmental Engineering, 3(5), 207-212.
Mishra, B. K., Priya, T., Gupta, S. K., & Sinha, A. (2016). Modeling and characterization of natural organic matter and its relationship with the THMs formation. Global Nest J, 18(4), 803-816.
Nissinen, T. K., Miettinen, I. T., Martikainen, P. J., & Vartiainen, T. (2002). Disinfection by-products in Finnish drinking waters. Chemosphere, 48(1), 9-20.
Qiang, Z., & Adams, C. D. (2004). Determination of monochloramine formation rate constants with stopped-flow spectrophotometry. Environmental Science & Technology, 38(5), 1435-1444.
Rathbun, R. E. (1996). Regression equations for disinfection by-products for the Mississippi, Ohio and Missouri rivers. Science of the Total Environment, 191(3), 235-244.
Reiff, F. M. (1995). Balancing the chemical and microbial risks in the disinfection of drinking water supplies in developing countries. IAHS Publications-Series of Proceedings and Reports-Intern Assoc Hydrological Sciences, 233, 23-30.
Richardson, S. D. (1998). Environmental analysis and remediation. Wiley Encyclopedia Series in Environmental Science, New York, pp. 1398-1421.
Richardson, S. D. (2005). New disinfection by-product issues: emerging DBPs and alternative routes of exposure. Global Nest J, 7(1), 43-60.
Rodriguez, M. J., Milot, J., Sérodes, J. B., & Pacaud, A. (2002). Estimation of bench-scale chlorine decay in drinking water using nth-order kinetic and back propagation: Neural Network Models. Water Quality Research Journal, 37(3), 613-635.
Rodriguez, M. J., Sérodes, J., & Morin, M. (2000). Estimation of water utility compliance with trihalomethane regulations using a modelling approach. Journal of Water Supply: Research and Technology - AQUA, 49(2), 57-73.
Rook, J. J. (1974). Formation of haloforms during chlorination of natural waters. Jour. Water Treatment Examinations, 23, 234-243.
Scully, F. E., Hartman, A. C., Rule, A., & LeBlanc, N. (1996). Disinfection interference in wastewaters by natural organic nitrogen compounds. Environmental Science & Technology, 30(5), 1465-1471.
Serajuddin, M., Chowdhury, A., & Hussain, M. M. (2018). Increasing level of ammonia in the surface raw water source at Dhaka, Bangladesh. Pollution, 4(2), 211-225.
Singer, P. C. (1994). Control of disinfection by-products in drinking water. Journal of Environmental Engineering, 120(4), 727-744.
Singer, P. C., & Reckhow, D. A. (1999) Chemical oxidation. in R. D. Letterman (Ed.), Water quality and treatment, (5th ed.). American Water Works Association, McGraw- Hill, New York.
Sohn, J., Amy, G., Cho, J., Lee, Y., & Yoon, Y. (2004). Disinfectant decay and disinfection by-products formation model development: chlorination and ozonation by-products. Water Research, 38(10), 2461-2478.
Sun, Y-X., Wu, Q-Y., Hu, H-Y., Tian, J. (2009), Effect of ammonia on the formation of THMs and HAAs in secondary effluent chlorination. Chemosphere, 76(2009), 631–637.
Stevens, A. A., & Symons, J. M. (1977). Measurement of trihalomethane and precursor concentration changes. Journal of American Water Works Association, 546-554.
Tieu, V. L., Chrlstophgr, J. P., & Roger, P. (1982), Influence of bromide and ammonia upon the formation of trihalomethanes under water-treatment conditions. Environ. Sci. Technol., 16(8), 1962 473.
USEPA. (1979). National interim primary drinking water regulations: Control of trihalomethanes in drinking water. Final rules. Fed. Reg, 44.
USEPA. (1999). Microbial and disinfection by-product rules - Simultaneous compliance guidance manual. United States Environmental Protection Agency, EPA 815-R-99-015, 4637-44.
Westerhoff, P., Debroux, J., Amy, G. L., Gatel, D., Mary, V., & Cavard, J. (2000). Applying DBP models to full‐scale plants. Journal‐American Water Works Association, 92(3), 89-102.
WHO. (1996). Ammonia in drinking-water: background document for development of WHO guidelines for drinking-water quality (No. WHO/SDE/WSH/03.04/01). World Health Organization.
Yang, X., & Shang, C. (2004). Chlorination byproduct formation in the presence of humic acid, model nitrogenous organic compounds, ammonia, and bromide. Environmental Science & Technology, 38(19), 4995-5001.
Yang, X., Shang, C., & Westerhoff, P. (2007). Factors affecting formation of haloacetonitriles, haloketones, chloropicrin and cyanogen halides during chloramination. Water Research, 41(6), 1193-1200.
Published
How to Cite
Issue
Section
ARK
License
Copyright (c) 2025 Md. Serajuddin, Aktarul Islam Chowdhury, Mehedi Hasan, Ehteshamul Haque

This work is licensed under a Creative Commons Attribution 4.0 International License.
Research Articles in 'Applied Science and Engineering Journal for Advanced Research' are Open Access articles published under the Creative Commons CC BY License Creative Commons Attribution 4.0 International License http://creativecommons.org/licenses/by/4.0/. This license allows you to share – copy and redistribute the material in any medium or format. Adapt – remix, transform, and build upon the material for any purpose, even commercially.










